The Neuroscience of Sigma-1 (σ1) And Its Relevance for Cognition Enhancement
Contributors
Author/Editor @arche
This post will talk about Sigma-1 (σ1) and its relevance for neurological disorders and its potential for high-level cognitive enhancement.
S1R bind with high affinity to several classes of chemically unrelated ligands such as neurosteroids, neuroleptics, DXM, and several psychostimulants such as cocaine, methamphetamine, MDMA and methacathinone. Consequently, it is thought that S1R may mediate the immunosuppressant, antipsy- chotic and neuroprotective effects of many drugs.
S1Rs regulate a number of neurotransmitter systems, including the glutamatergic, dopaminergic [DA], serotonergic, noradrenergic and cholinergic systems.
Sigma-1 receptors are expressed in neurons and glia and act as molecular chaperones that regulate various cellular processes important for cognitive function. These include calcium signaling, neurotransmitter release (especially of acetylcholine and glutamate), and mechanisms underlying neuroplasticity. Unlike many other neurotransmitter receptors that show declining density in the aging brain, sigma-1 receptor density is preserved or even increased with age. However, in pathological conditions like Alzheimer's disease and to some degree Parkinson's disease, there is a noticeable reduction of sigma-1 receptors.
Indirect regulation of transcriptional activity by S1R contributes to its neuroprotective properties. For example, S1R may prevent neuronal death by upregulating expression of the antiapoptotic mitochondrial protein Bcl-2 [1][2].
S1R facilitates NMDA receptor signaling and neurotransmission in hippocampal neurons [3][4][5], possibly through altering responses to calcium signals (e.g., inhibiting Ca2+-activated SK channels) and promoting expression of NMDA receptor subunits and their trafficking to the plasma membrane [6][7]. S1R can also obviate/remove negative-regulation of NDMARs by cannabinoid 1 receptor (CB1R) [8]. These interactions enhance neuronal firing and maturation of mushroom spines from NMDA receptor activation [3][6][7]. Modulation of calcium signaling by S1R may regulate synaptic plasticity through stimulation of CaMKII, PKC, and ERK (Moriguchi et al., 2011).
Sigma-1 Agonists 
Many S1R agonists are anti-amnestic, synaptogenetic, and neuroprotective in conditions of neuronal stress. They also mitigate disease and symptoms in experimental models of ALS, Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), stroke, and TBI.
S1R agonists promote neurogenesis in the hippocampus [10] and they may mitigate memory impairment because they can stabilize mature, mushroom spines [11], which serve as sites of robust synaptic connections encoding lasting information [12][13].
They also appear to activate TrkB both through BDNF-dependent [14] and independent mechanisms [15]. This may involve regulation of BDNF expression and processing as well as direct interactions of S1R with the TrkB receptor [16][14][15].
Sigma-1 agonists have been found to improve cognitive function in a wide variety of animal models related to cholinergic dysfunction, NMDA receptor hypofunction, amyloid beta toxicity, aging, hypoxia, prenatal stress, and other conditions. The cognitive enhancement by sigma-1 agonists in these models is mediated via sigma-1 receptors. Proposed mechanisms underlying these pro-cognitive effects include facilitating the release of acetylcholine and glutamate, regulating NMDA receptor signaling, modifying calcium homeostasis, and promoting neuronal differentiation and plasticity.
Some antidepressant/anti-anhedonic medications and Alzheimer's medications like donepezil also happen to act as Sigma-1 receptor agonists, and this action likely contributes to their therapeutic effects on cognition. In summary, Sigma-1 receptors play an important neuromodulatory role in various processes fundamental to learning and memory. Sigma-1 agonists continue to show promise as cognitive enhancers, especially under pathological conditions involving cholinergic or glutamatergic deficits.
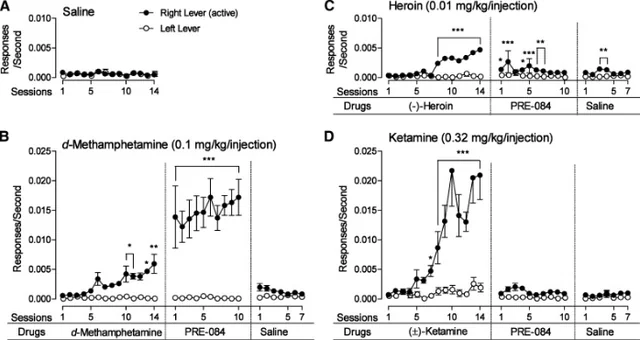
The problem with Sigma-1 agonism is that it has some issues. First, it can be potentially reinforcing [17][18], with Sigma-1 agonists being potentially addictive if co-administered with compounds which enhance dopamine release. Sigma-1 agonists also effect locomotor activity, which is not an optimal profile for a compound if high selectivity is the target. Agonism can also potentially cause immunosuppression [19].
Allosteric Sig1R Modulators 
The first evidence indicating that a compound demonstrates allosteric activity on Sig1R came from radioligand binding studies. The first drug discovered as an allosteric modulator of Sig1R was phenytoin, an anti-convulsant drug that primarily acts by blocking the voltage-gated sodium channels. Phenytoin sensitivity was considered an intrinsic characteristic of the sigma-1 subtype of sigma sites, and Sig1R were defined mainly through their high-affinity sites for the dextrorotatory isomers of benzomorphans and their sensitivity to phenytoin [20].
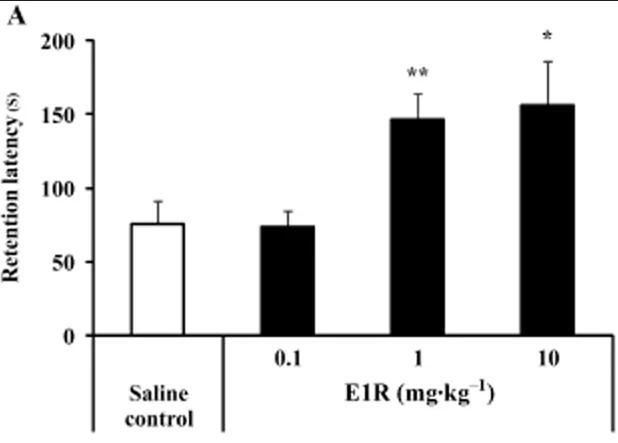
Methylphenylpiracetam (E1R) was discovered to target only the Sig1R site in in vitro pharmacological profiling assays. E1R has been shown to alleviate scopolamine-induced cognitive impairment in mice, as assessed using passive avoidance and spontaneous alternation tests. The effects of E1R were antagonized by the selective Sig1R antagonist NE-100, suggesting a Sig1R positive allosteric modulatory effect in vivo.
E1R is a unique racetam compound because it displays cognition enhancements linked to positive allosteric sigma-1 receptor modulation. E1R is the first piracetam derivative reported to modulate sigma-1 receptors.
The stereochemistry of allosteric Sig1R modulators is an important factor in their activity. For example, E1R is a 4R,5S-isomer of methylphenylpiracetam, and it has been shown to be a selective PAM of Sig1R. However, its 4S,5R-isomer, called UN101063, has no Sig1R activity. Therefore, the stereochemistry of allosteric Sig1R modulators needs to be carefully considered in the design and optimization of novel compounds.
E1R, being based on phenylpiracetam, has a high predicted ADMET safety profile and a low predicted dose. A key advantage of E1R over other Sigma-1 receptor modulators is its lack of effects on locomotor activity. Therefore, E1R represents a promising lead compound for further development as a therapeutic agent, particularly for treating symptoms of cognitive disorders and neurodegenerative diseases. The only problem with E1R is of its difficulty to produce due to its stereoisomer configuration.
Among all the positive allosteric Sig1R modulators described, E1R, OZP002, and fenfluramine showed Sig1R-dependent memory-improving effects [21][22][23]. E1R, however, is the only modulator showing dose-dependent memory-improving activity in drug-naïve animals [21].
There is also SOMCL-668, which is a PAM of Sigma-1, however E1R seems to have a better pharmacological profile, as it has not been demonstrated that SOMCL-668 improves memory and cognition through a Sig1R-related pathway.
Sig1R involvement in psychedelic neurology 
One study [24] found that indole-N-methyl transferase (INMT), an enzyme that converts tryptamine into the sigma-1 ligand dimethyltryptamine (DMT), is also localized to postsynaptic sites of C-terminals in close proximity to the S1R. This close association of INMT and S1Rs suggest that DMT is synthesized locally to effectively activate S1R in MN (motoneurons).
Sigma-1 seems quite important for the effects of DMT (and other psychedelics), which is quite interesting considering the most studied pathways for psychedelics are seretonin subunits (e.g 5-HT2A). This is most likely because DMT is a potent agonist at Sigma1.
In one study [25] Sig-1R knockout mice, which reacted normally to the locomotor stimulating effect of methamphetamine, did not become hyper-active in response to DMT. This shows a large part of its effects are most likely mediated just through Sigma1.
This area is relatively under-researched, and warrents more investigation.
Potential Ago-Allosteric experimentation 
When a Sigma-1 agonist and PAM (Positive Allosteric Modulator) are combined together, they create what is known as a "ago-allosteric" or "superallosteric". Because the main binding site and allosteric binding site are seperate, they can both potentiate each other without conflicting. This can lead to an even greater response than normally possible with either.
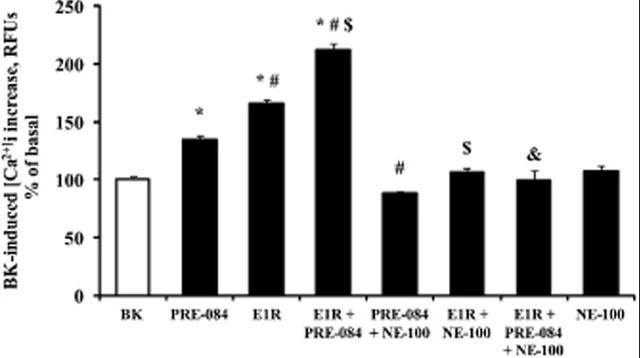
E1R (Sigma-1 Agonist) + E1R (Sigma-1 PAM) are a good example of a functional ago-allosteric. It was shown that both selective Sig1R agonist PRE-084 and allosteric modulator E1R increased the BDK-induced [Ca2+]i increase, while the combination of both compounds resulted in an even more pronounced cellular response [21].
Discussion 
Modulating Sigma-1, especially with PAMs, seems like a very promising mechanism of action for cognitive enhancement and also for treating numerous existing neurological disorders. Using allosterics rather than agonists seems to be the way to go, as they have a superior effect profile and efficacy.
A lot of existing pharmacological compounds are ligands at Sigma-1, however they lack selectivity and also lack allosteric affinity. Compounds with high selectivity and also with affinity for the allosteric site only are most likely superior candidate compounds.
The Sigma-1 PAM, E1R, has demonstrated a high preclinical efficacy in terms of increasing the retention latency (short-term memory) of passive avoidance in mice. Other allosterics, such as SOMCL-668 have also shown efficacy in studies.
Thanks for reading.