M1 Allosteric Enhancement And The Potential of Muscarinic Receptors
Contributors
Author/Editor @arche
Muscarinic receptors have been an interesting area of research personally for a while now, with interesting results depending on how they are modulated. Muscarinic acetylcholine receptors are activated by binding to acetylcholine released from the presynaptic neurons.
This post will discuss allosteric modulation (aka "PAM'ing") of Muscarinic M1, or just known as M1R/M1.
The reason for the discussion around allosteric modulation is because M1 agonism is undesirable due to side effects, limited efficacy, and other issues.
M1R is predominantly expressed at post-synaptic terminals of frontal cortex and hippocampus and has a vital role in learning, long-term memory formation, and synaptic plasticity [6]. It is mostly located in pyramidal cells [8], which have relevance for spatial cognition.
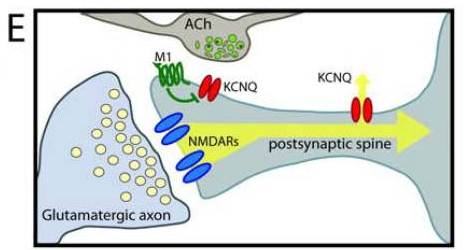
The other Muscarinic Receptors (M2-5) are mainly located in lower cognitive areas like the corpus striatum, hypothalamus, substantia nigra pars compacta, ventral tegmental area, etc. Due to this difference in location, M1R seems like a more relevant target for high-level cognition.
M1 PAMs are neuroprotective, slowing prion neurodegeneration [7]. They also most likely reduce tau [8]. M1 Stimulation with PAMs also increases delay-cell firing in the dlPFC [9], which you may have heard mentioned before in other posts as very important for working memory. M1 modulators may pair well with mGluR3 enhancement and other approaches that enhance delay-cell firing, as a way to enhance working memory even further.
Through interaction with muscarinic M1/M3 post-synaptic receptors, extracellular tau is able to reciprocally induce degeneration of the basal forebrain cholinergic neurons. [8]
M1 Receptors are expressed postsynaptically on spines in dlPFC and also is found on Golgi [10]. One thing to note is that Muscarinic M1R overstimulation disrupts working memory activity in the PFC [12], so increasing endogenous activation rather than creating an artificial overstimulation is definitely ideal, especially if you want higher-brain circuits to be 'well-tuned'.
We propose that transient ACh release in the cortex, as occurs in response to salient environmental cues (Parikh et al. 2007), may help focus attention on relevant sensory input by transiently driving populations of pyramidal neurons toward a common hyperpolarized membrane potential via M1‐receptor‐driven calcium release from intracellular stores and SK channel activation. Such hyperpolarization may serve to ‘reset’ synaptic integration across populations of pyramidal neurons, such that future action potential generation more accurately reflects updated, rather than previous, patterns of afferent input. [11]
Candidate Molecules 
It is critical to optimize PAMs that maintain a strict dependence of receptor signaling on activation of receptors by endogenous transmitters. Even PAMs with some agonist affinity (aka "Ago-PAMs") still have the downsides normal agonists do, so candidate molecules need to have near to no agonist affinity at effective doses.
TAK-071 
TAK-071 is an M1 PAM candidate developed by Takeda, and it is a quite well-rounded molecule. It has an EC50 for the allosteric site of 520 nM [4], and it is most likely the best candidate for M1 PAM out of molecules researched. It does not have agonism affinity at effective doses and it has been well tolerated in clinical safety testing.
Overall, TAK‐071 was safe and well tolerated at the doses tested. There were no serious AEs or deaths. [5]
The half life is long at 45-60 hours in humans [5], and its estimated effective dose in humans is 2-4mg.
MK-7622 
MK-7622 lacks ideal potency (a repeating pattern with Merck's molecules) and efficacy as a M1 PAM. It also has agonist affinity (an "Ago-PAM") [1], inducing robust behavioral convulsions, defeating the upside of allosteric modulation as the agonism outweighs the benefits.
VU- Molecules 
There are a few VU- M1 PAMs (such as VU0119498, VU0451725, VU0550164, etc.), however VU0453595 (VU595) is mentioned a lot accross studies. It is selective for the allosteric site, with very minimal agonism. The molecule has some downsides though, being not that potent (EC50=2140 nM [2]), with an approximate dose of 30mg in humans.
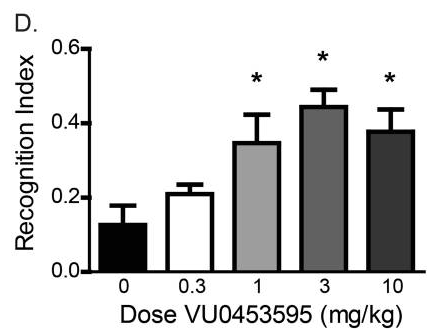
VU0453595, which lacks agonist activity, induces a robust improvement in recognition memory in healthy adult rats assessed using the novel object recognition task. At 1, 3, and 10 mg/kg, VU0453595 enhances object recognition as indicated by a significant increase in the recognition index (Dunnett’s multiple comparison test: p = 0.0008) [1]
A newer M1 PAM known as VU0486846 was also developed [3], with an improved potency (EC50=300 nM). Saying that, weirdly the cognitive difference at 1mg/kg and 3mg/kg in rats was actually quite similar and seemingly more effective at 1mg/kg for VU0453595. This could be due to lowered CNS penetration or something else.
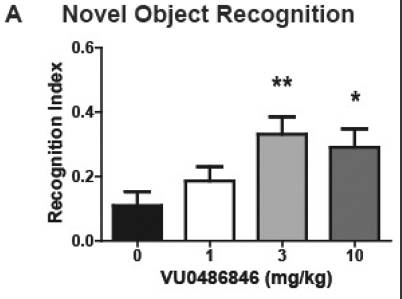
VU0486846 is also more complex in terms of molecular structure, with modified chirality likely making it a difficult synthesis. Due to this, it is not really an ideal candidate, in comparison to something like TAK-071.
PF-06764427 
PF-06764427 has agonist affinity, making it a sub-par candidate [1]. There are other PF molecules, but most of them have poor pharmacokinetics, so they lack interest.
Discussion 
Enhancing Muscarinic M1 through the allosteric site proves to be a very interesting mechanism for cognitive enhancement, improving healthy models and models of neurodegeneration. It deserves more research and investigation, and it honestly is an underrated focus.
Out of the candidate M1 PAMs provided, TAK-071 and VU0453595 are the most ideal, with the Takeda molecule likely being the best out of the candidates.
Combined with other delay-cell firing enhancers, the working memory & spatial cognition enhancement from M1R PAMs may be highly significant, and may contribute to a key factor in enhancing cognition in health and unhealthy individuals.
If you like this post, Follow us on X (Twitter) @PenchantLabs, and share this post. :)